REXEED SX - Single Use, High Flux, Dry Dialyzers
Asahi's unique membrane technology results
in less stimulation to blood components.
Asahi has achieved a new level of overall performance in dialyzer design by combining the new polysulfone membrane REXBRANE™ with a new jacket design allowing for optimum flow dynamics. Incorporated on the inner surface is Asahi's unique hydrophilic gel layer technology. This minimizes blood membrane interaction and plays a crucial role in anti-coagulation requirements.
| • Surface areas from 1.5m2 - 2.5m2 | • REXBRANE membrane |
| • X- jacket design for improved flow dynamics | • ETO free and E-Beam sterilized |
| • Exceptional toxin removal | • Efficient heparin utilization |
| • Required priming volume reduced to 500mL | • Inner gel layer reduces platelet activation |
- Specifications
- Performance
- SX Comparison
- Flow Dynamics
- Biocompatibility
- Priming Chart
- Products
REXEED SX Specifications
Print Specifications (PDF)
| REXEED-15SX | REXEED-18SX | REXEED-21SX | REXEED-25SX | |
| Inside Diameter of Hollow Fiber (um) | 185 | |||
| Wall Thickness of Hollow Fiber (um) | 35 | |||
| Effective Surface Area (m2) | 1.5 | 1.8 | 2.1 | 2.5 |
| Priming Volume (mL) | 88 | 100 | 119 | 137 |
| Max. TMP | 66kPa (500 mmHg) | |||
| Dimensions (mm[L] x mm [DJ]) | 334(L) x 38(D) | 334(L) x 41(D) | 334(L) x 43(D) | 334(L) x 47(D) |
| Sterilization | Electron Beam | |||
Fine Tuned Technology
E-Beam Serialization - Electron-beam sterilization, a newly developed technology, is used in REXEED SX dialyzers.
Color-Coded Headers - Red / Blue color coded headers are used for easier distinction when connecting REXEED SX dialyzers to a dialysis machine and blood tubing set.
Required Fluid Volume for Priming Reduced to 500 mL - Since the fluid volume required for priming is less using REXEED SX technology, the valuable time of medical personnel can be saved and the total cost of the dialysis treatment is reduced.
Reduced Size and Weight - The reduced size and weight of the models in the REXEED SX series afford easy handling.
REXEED SX Dialyzer Performance
Print Performance (PDF)
| Performance (in vitro) | |||||||||
| REXEED-15SX | REXEED-18SX | ||||||||
| Qd=500mL/min | Qd=800 mL/min | Qd=500mL/min | Qd=800 mL/min | ||||||
| Clearance(mL/min) | Qb=200 mL/min | Qb=300 mL/min | Qb=400 mL/min | Qb=400 mL/min | Qb=200 mL/min | Qb=300 mL/min | Qb=400 mL/min | Qb=400 mL/min | |
| Urea | 197 | 275 | 329 | 363 | 198 | 280 | 337 | 370 | |
| Creatinine | 193 | 261 | 303 | 339 | 195 | 268 | 313 | 349 | |
| Phosphate | 188 | 247 | 281 | 314 | 192 | 256 | 293 | 326 | |
| Vitamin B12 | 152 | 181 | 198 | 219 | 160 | 192 | 211 | 233 | |
| KOA (urea)(mL/min) | 1421 | 1576 | |||||||
| KUF(mL/hr/mmHg) | 75 | 82 | |||||||
| REXEED-21SX | REXEED-25SX | ||||||||
| Qd=500mL/min | Qd=800 mL/min | Qd=500mL/min | Qd=800 mL/min | ||||||
| Clearance(mL/min) | Qb=200 mL/min | Qb=300 mL/min | Qb=400 mL/min | Qb=400 mL/min | Qb=200 mL/min | Qb=300 mL/min | Qb=400 mL/min | Qb=400 mL/min | |
| Urea | 199 | 284 | 347 | 378 | 199 | 287 | 355 | 384 | |
| Creatinine | 197 | 275 | 326 | 361 | 198 | 281 | 335 | 369 | |
| Phosphate | 195 | 266 | 307 | 341 | 197 | 273 | 317 | 352 | |
| Vitamin B12 | 168 | 206 | 227 | 251 | 174 | 218 | 240 | 266 | |
| KOA (urea)(mL/min) | 1809 | 2052 | |||||||
| KUF(mL/hr/mmHg) | 93 | 104 | |||||||
KUF: with bovine blood, TP=60+-5 g/L. Hct.=32+-2%, Qb=300 mL/min • Clearance: Qf=0 mL/min • KoA: Qb=400 mL/min, Qd= 800mL/min, Qf=800 mL/min, Qf=0 mL/min
REXEED SX Series Comparison to US Competitors
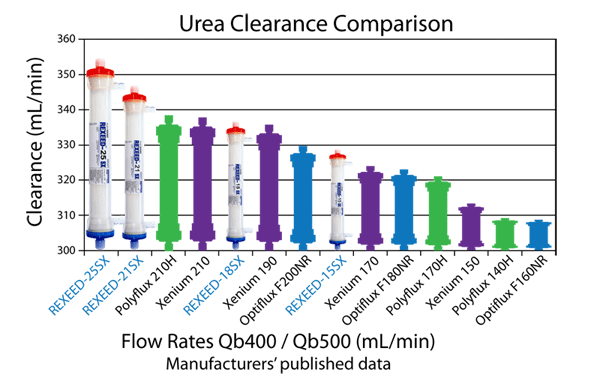
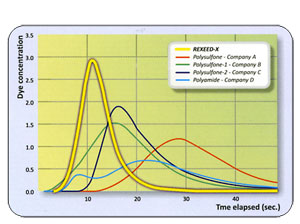
Urea Clearance Comparison
With regard to small molecule clearance, REXEED SX dialyzers showed the highest level of performance compared to other synthetic membrane dialyzers.
This excellent clearance is attributed to Asahi's superior spinning technology and optimization achieved by the membrane structure and the improved housing design of REXEED SX dialyzers.
Small molecule clearance: all data are as per the manufacturer's specifications for in vitro procedures. Data obtained from product catalog.
Performance in-vetro REXEED SX Series vs US Competitors - Print (PDF)
| Surface Area (m2) | Priming Volume (m2) | Clearance Qb300, Qd500 | Clearance Qb400, Qd500 | Clearance Qb400, Qd800 | KoA Urea | ||||||||||
| Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | ||||
| REXEED-15SX | 1.5 | 88 | 275 | 261 | 247 | 181 | 329 | 303 | 281 | 198 | 363 | 339 | 314 | 219 | 1421 |
| Optiflux F160NR | 1.5 | 83 | 266 | 238 | 230 | 152 | 308 | 264 | 275 | 157 | 344 | 295 | 296 | 173 | 1064 |
| Optiflux F180NR | 1.8 | 99 | 274 | 251 | 238 | 168 | 323 | 286 | 287 | 178 | 358 | 316 | 314 | 203 | 1239 |
| Polyflux 140H | 1.4 | 94 | 262 | 232 | 220 | 149 | 309 | 266 | 250 | 163 | - | - | - | - | 1040 |
| Polyflux 170H | 1.7 | 115 | 270 | 243 | 232 | 162 | 321 | 281 | 266 | 178 | - | - | - | - | 1190 |
| Xenium 150 | 1.5 | 91 | 269 | 251 | 233 | 174 | 313 | 285 | 262 | 192 | 349 | 324 | 296 | 218 | 1123 |
| Surface Area (m2) | Priming Volume (m2) | Clearance Qb300, Qd500 | Clearance Qb400, Qd500 | Clearance Qb400, Qd800 | KoA Urea | ||||||||||
| Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | ||||
| REXEED-18SX | 1.8 | 100 | 280 | 268 | 256 | 192 | 337 | 313 | 293 | 211 | 370 | 349 | 326 | 233 | 1576 |
| Optiflux F180NR | 1.8 | 99 | 274 | 251 | 238 | 168 | 323 | 286 | 287 | 178 | 358 | 316 | 314 | 203 | 1239 |
| Optiflux F200NR | 20 | 112 | 277 | 253 | 250 | 173 | 330 | 289 | 290 | 189 | 364 | 325 | 319 | 206 | 1317 |
| Polyflux210H | 2.1 | 125 | 281 | 259 | 249 | 183 | 339 | 303 | 289 | 203 | - | - | - | - | 1500 |
| Xenium 170 | 1.7 | 99 | 274 | 259 | 240 | 185 | 324 | 293 | 269 | 202 | 359 | 330 | 300 | 225 | 1239 |
| Surface Area (m2) | Priming Volume (m2) | Clearance Qb300, Qd500 | Clearance Qb400, Qd500 | Clearance Qb400, Qd800 | KoA Urea | ||||||||||
| Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | ||||
| REXEED-21SX | 2.1 | 119 | 284 | 275 | 266 | 206 | 347 | 326 | 307 | 227 | 378 | 361 | 341 | 251 | 1809 |
| Optiflux F200NR | 2.0 | 112 | 277 | 253 | 250 | 173 | 330 | 289 | 290 | 189 | 364 | 325 | 319 | 206 | 1317 |
| Polyflux210H | 2.1 | 125 | 281 | 259 | 249 | 183 | 339 | 303 | 289 | 203 | - | - | - | - | 1500 |
| Xenium 190 | 1.9 | 114 | 282 | 269 | 250 | 197 | 336 | 315 | 288 | 218 | 369 | 349 | 320 | 247 | 1487 |
| Xenium 210 | 2.1 | 126 | 285 | 272 | 253 | 200 | 338 | 316 | 293 | 220 | 371 | 352 | 325 | 249 | 1614 |
| Surface Area (m2) | Priming Volume (m2) | Clearance Qb300, Qd500 | Clearance Qb400, Qd500 | Clearance Qb400, Qd800 | KoA Urea | ||||||||||
| Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | Urea | Creatinine | Phosphate | Vit B12 | ||||
| REXEED-25SX | 2.5 | 137 | 287 | 281 | 273 | 218 | 355 | 335 | 317 | 240 | 384 | 369 | 352 | 266 | 2052 |
| Optiflux F200NR | 2.0 | 112 | 277 | 253 | 250 | 173 | 330 | 289 | 290 | 189 | 364 | 325 | 319 | 206 | 1317 |
| Polyflux210H | 2.1 | 125 | 281 | 259 | 249 | 183 | 339 | 303 | 289 | 203 | - | - | - | - | 1500 |
| Xenium 210 | 2.1 | 126 | 285 | 272 | 253 | 200 | 338 | 316 | 293 | 220 | 371 | 352 | 325 | 249 | 1614 |
Building a Better Dialyzer
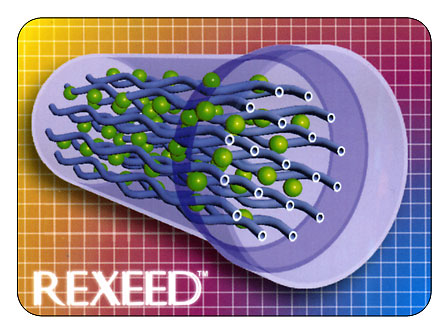
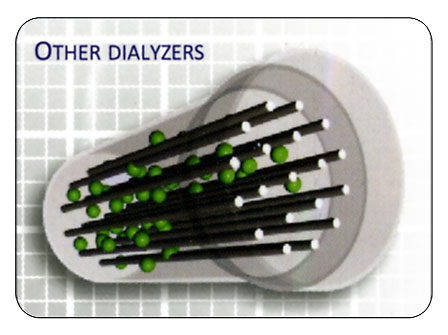
Flow Distribution of Dialysate
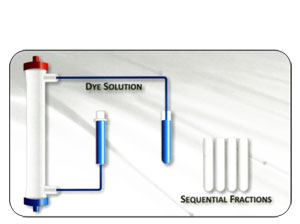
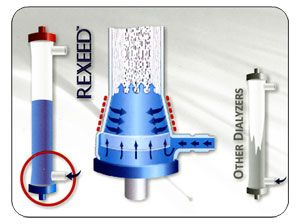
Superior Biocompatibility - Less Stimulation to Blood Components
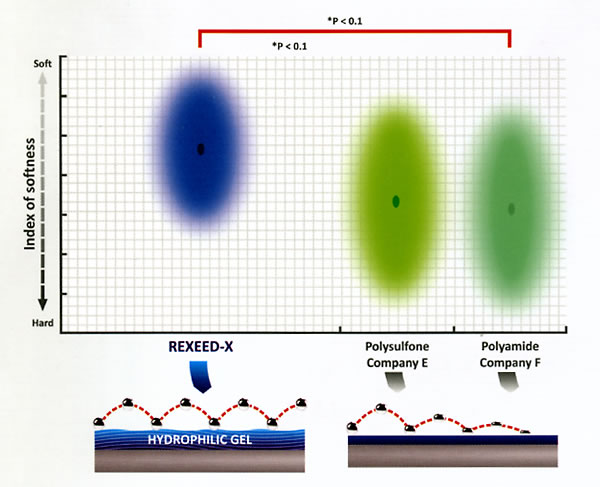
On exposure to water, the hydrophilic gel layer formed on the inner surface of the REXEED SX membrane is softer than that formed in the case of other membranes, as shown in the graph below.
This is also one of the major salient features of REXEED SX. A softer inner surface helps to alleviate blood-membrane interaction.
Softness Of The Hydrophilic Layer On The Surface of Internal Membrane
Each membrane was incubated in distilled water for 30 minutes, and then, force-distance curve measurements using Atomic Force Microscopy (AFM) were performed to ensure softness of the inner surface of the membrane.
Each parameter was measured 100 times at different points, and then, the average and standard deviation were calculated.
The major axis of each ellipse shows the standard deviation and the center shows the average value.
The above diagrams are schematic representations of the platelet-membrane interaction. If platelets come in direct contact with the membrane surface, they may be activated, leading to adhesion to the membrane surface. The coagulation system is also highly susceptible to such activation.
The hydrophilic gel layer on the REXEED SX membrane inner surface is designed to suchion the platelets and other blood components from activation caused by blood-membrane interaction.
REXEED SX Priming Chart
Print Priming Chart (PDF)
It is important to remember that this diagram is intended to be used as a quick reference guide.
Please refer to the IFU enclosed in each carton of dialyzers for more detailed information.
It is important to use aseptic technique throughout this procedure.
 STEP 1:
STEP 1:
Position the dialyzer on the machine with the venous header (blue) up.
 STEP 2:
STEP 2:
Place arterial and venous bloodlines on the machine according to manufacturer's IFU. Close all small clamps.
 STEP 3:
STEP 3:
Connect 1-liter bag of 0.9% saline to the I.V. administration set.
Note: Saline volume may be determined by unit policy.
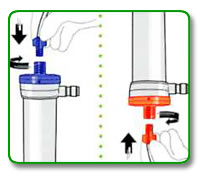 STEP 4:
STEP 4:
Aseptically attach both arterial and venous bloodlines to appropriate dialyzer connector. (arterial is color-coded red and venous is color-coded blue).
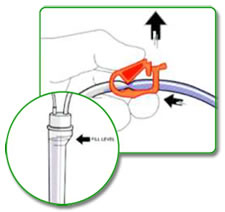 STEP 5:
STEP 5:
Open saline clamps and allow patient end of arterial line to fill with saline by gravity. Please note that all drip chambers should be maintained at approximately 3/4 full depending on manufacture's IFU. Close bloodline arterial tubing clamp.
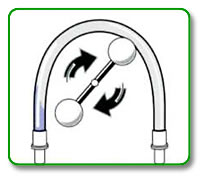 STEP 6:
STEP 6:
Open all necessary clamps and start blood pump at slow flow rate (approximately 100mL.min) to prime arterial bloodline, dialyzer and venous bloodline. Be sure to fill drip chambers (if present) to appropriate levels to avoid saline-air mix in bloodline. Ensure that all air is removed from blood pump segment.
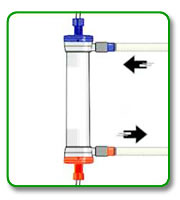 STEP 7: Attach dialysate lines to the dialyzer and begin dialysate flow with minimum UF.
STEP 7: Attach dialysate lines to the dialyzer and begin dialysate flow with minimum UF.
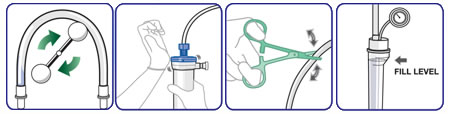 STEP 8: Briefly pinch and release either the arterial or venous blood tubing near the dialyzer to assist in purging residual air from the fibers. (this process may be continued throughout the priming process until air fails to accumulate in the header).
STEP 8: Briefly pinch and release either the arterial or venous blood tubing near the dialyzer to assist in purging residual air from the fibers. (this process may be continued throughout the priming process until air fails to accumulate in the header).
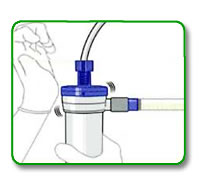 STEP 9:
STEP 9:
GENTLY tilt and tap the venous dialyzer header to remove accumulated air. (Remember that it is possible to remove air only when the dialyzer is in the venous header up position))
STEP 10:
Continue priming process until 500 mL total saline has passed through the dialyzer and no further air can be removed. Note: Saline volume may be determined by unit policy. If air bubbles remain, continue priming with additional saline until clear. Replace empty saline bag.
Step 11:
This completes priming of the ASAHI dry-packed dialyzer. Treatment may begin, or, if it is normal unit procedure, it is possible to recirculate saline until treatment begins by connecting the patient ends of the arterial and venous bloodlines, opening the saline clamp and resuming blood pump at a slow rate. As treatment begins, dialyzer may be rotated arterial end up. 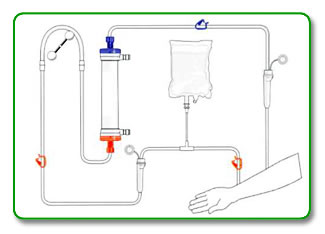
REXEED SX
| REXEED SX - Single Use, High Flux, Dry Dialyzers | |||||||||
| Product | Size M2 | Priming Volume |
Use | Type | Membrane | Sterilization | KOA (Urea) |
||
| REXEED 15SX | 1.5 | 88 | Single | Dry | Polysolfone | E-Beam | 1421 | ||
| REXEED 18SX | 1.8 | 100 | Single | Dry | Polysolfone | E-Beam | 1576 | ||
| REXEED 21SX | 2.1 | 119 | Single | Dry | Polysolfone | E-Beam | 1809 | ||
| REXEED 25SX | 2.5 | 137 | Single | Dry | Polysolfone | E-Beam | 2052 | ||
